What is mass spectrometry?
Mass spectrometry is a method to measure the molecular weights (masses) of individual molecules that have been converted to electrically charged ions. A mass spectrometer in the clinical laboratory is used to:
1. Identify molecules present in solids, liquids, and gases.
2. Determine the quantity of each type of molecule.
There are four components of every mass spectrometer (inlet system, ion source, mass analyzer and detector).
What is an internal standard?
In mass spectrometry, where a quantitation of the molecule of interest is desired, an internal standard is included for two reasons:
1. It accounts for any variation which happens in the many sample preparation steps done before actual mass analysis is done.
2. It provides a source of comparison between the molecule of interest, whose concentration is unknown, and a chemically identical molecular isotope form of the molecule, whose concentration is known.
How does an ion source on a mass spectrometer create ions?
There are two methods that can be used to create ions. The first is called a gas phase technique. This technique is used for molecules that are naturally volatile (like alcohols) and are thermally stable. The mass spectrometer volatilizes the sample first, and then ionizes the sample's molecules.
The second technique is called desorption. This is used for molecules that are not naturally volatile or ionized, which actually includes most of the molecules of clinical interest (drugs, vitamins, amino acids, fatty acids, and steroids). "Desorption" means to lift off of a surface. There are two common kinds of desorption ion sources: Electrospray ionization and Matrix- Assisted Laser Desorption Ionization (MALDI).
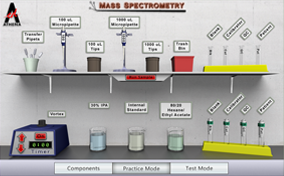 A common problem faced by many laboratory technicians is to determine what and how much of a substance is in a sample of material. Mass spectrometry is one methodology used to help make this determination. Drug analysis, protein assay, and volatile identification are just a few of the important processes that use mass spectrometry. Are you ready to move mass to identify an unknown using spectral analysis?
A common problem faced by many laboratory technicians is to determine what and how much of a substance is in a sample of material. Mass spectrometry is one methodology used to help make this determination. Drug analysis, protein assay, and volatile identification are just a few of the important processes that use mass spectrometry. Are you ready to move mass to identify an unknown using spectral analysis?)


