What are elements?
Elements are the primary substance from which all other materials are built. A substance that cannot be broken down into simpler substances by chemical means is called an element. Over 100 different elements have been identified. Most of these elements exist on the earth in different amounts. Gold, silver, oxygen, and sodium are just of few of the many important elements that you may already be familiar with. The periodic table provides a visual representation and listing of the known elements. The smallest part of an element that still has all of the properties of the element is an atom.
What is a compound?
A compound is a pure substance that is composed of two or more elements that have been chemically joined. A compound can only be broken down into its elements by chemical means. Water, sugar, table salt, and carbon dioxide are just a few of the compounds that you might have experienced in your life. The smallest part of a compound that still has all of the properties of the compound is a molecule.
What are chemical symbols?
Scientists can get just as tired of writing out element names as you can, so they created a shorter way of representing elements. Chemical symbols provide a shorthand way of writing the name of an element. Examples include "O", the symbol for oxygen, "N" for nitrogen, and "Mg" for magnesium. The periodic table provides a list of the chemical symbols and element names.
What is a chemical formula?
Compounds need a form of short-hand just like elements do, so chemical formulas were created to save ink when writing out long names. A chemical formula states the number and kind of each element present in one molecule of a compound. For instance, table sugar has the chemical formula of C12H22O11. This means that three different elements are present in sugar: carbon (C), hydrogen (H), and oxygen (O). The number 12 just to the right of the "C" in the formula states that one molecule of sugar has 12 atoms of carbon (C). Each sugar molecule also has 22 atoms of hydrogen (H) and 11 atoms of oxygen (O). These numbers, 12, 22, and 11, are called subscripts. Subscripts appear to the right and a little bit lower than the element that the subscript goes with. If no number appears to the right of an element, the subscript is assumed to be one.
What is the Law of Definite Proportions?
This law states that a pure sample of a given compound will always have the same proportion by mass of the elements from which it is formed. In others words, any sample of pure water on Earth or in the universe would have the same chemical formula, H2O. This states that for every one oxygen atom (with a mass of 16 amu), there needs to be two hydrogen atoms (with a total mass of 2 amu) to form the compound called water. For instance, if a substance had the chemical formula H2O2, it wouldn't be water. In fact, this compound is hydrogen peroxide, which is a chemical used to bleach hair blond.
What are covalent compounds?
Elements combine to form compounds in two major ways: either by ionic or covalent bonding. Atoms share electrons in covalent compounds. The two or more atoms share an electron or electrons to try to end up with the same arrangement of electrons as the noble gases. The noble gases are extremely stable and react with very few substances. Non-metals usually combine with themselves or other non-metals to form covalent compounds. With the exception of the noble gas helium, most other noble gases are stable because they have eight electrons in their outer level.
Remember, elements combine on their own to form compounds that are more stable.
What are ionic compounds?
Elements combine to form compounds in two major ways: either by ionic or covalent bonding. Ions, or atoms that have gained or lost an electron or electrons, combine to make ionic compounds. An electron or electrons must be completely moved from one atom to another atom in this process. The atom that loses the electron becomes positively charged, while the electron-gaining atom ends up with a negative charge. After the electron has been transferred, the ions "stick" together as a result of the positive ion having an attraction for the negative ion. Generally, the active metals and the active non-metals combine to form ionic compounds.
Remember, elements combine on their own to form compounds that are more stable. When an atom loses or gains an electron to form an ion, the ion usually has the same arrangement of electrons as the noble gases.
What is the Periodic Table?
The periodic table provides a visual representation of the grouping of elements by common physical and chemical properties. The periodic table has elements arranged by increasing atomic number. Over the years, a number of scientists have added insights and ideas to the currently used versions of the periodic table.
What are families (or groups) on the Periodic Table?
The vertical columns (going up and down) on the periodic table are called families, or groups. Families contain elements with similar chemical and physical properties. The periodic table has 18 families.
An easy way to find out how many electrons the element has in its outer level is to simply look at the family number. For instance, lithium (Li) and sodium (Na) are in the IA family, and they each have one electron in the outer level. Oxygen is a VIA, so all oxygen atoms have six electrons in their outer level.
What are periods (or rows) on the Periodic Table?
The horizontal rows (going from one side to the other side) on the periodic table are called periods. Periods contain elements that tend to have more different chemical and physical properties as the distance separating the elements on the periodic table increases. The periodic table has seven periods.
 Many chemists want to make new substances from given ingredients. Chemists must use the right amount of each material and complete the reaction using specific conditions, such as temperature and pressure. Your challenge is to make substances from their chemical components by combining the elements in the correct amounts. Are you ready to start mixing?
Many chemists want to make new substances from given ingredients. Chemists must use the right amount of each material and complete the reaction using specific conditions, such as temperature and pressure. Your challenge is to make substances from their chemical components by combining the elements in the correct amounts. Are you ready to start mixing?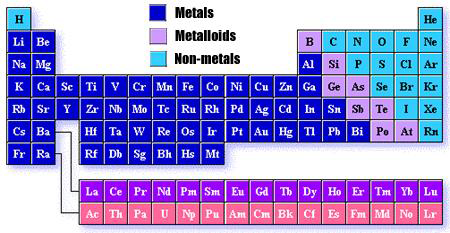)


