Here are some definitions to help you in your investigation.
Ignite - to light on fire or burn
Flask - a container to hold a liquid
Burn - to combine with oxygen gas and produce heat and light
Chemical Reaction - a process in which two or more substances change into a new, different substance or substances
Mass - the amount of matter in something
Energy - the ability to do work
Conversion - to change from one form to a different form
Conservation - the total value of something does not change because of some process
Conservation of Mass - the total mass that enters a chemical reaction must equal the total mass that exits the reaction
Transform – to convert one form of energy to a different form of energy
Transfer - to move heat energy from one place to another place
What is energy?
Energy is defined as the ability to do work. Work is a force acting over a distance, so energy is the ability to exert forces on different things. For example, pushing a box across a floor takes a certain amount of energy. The amount of energy necessary would depend on the mass of the box, the distance the box is being pushed and the type of surface that the box is being pushed on. Energy can take many different forms. Examples of different types of energy are mechanical energy, electrical energy, thermal (heat) energy, and sound energy.
What is a calorie?
A calorie is a unit of energy, or more specifically a unit of heat. A calorie, also called a gram calorie, is the amount of heat energy needed to increase the temperature of 1 gram of water by 1 °C. One calorie is equivalent to 4.2 joules. A Calorie (with a capital "C"), also called a kilogram calorie, is the amount of heat energy needed to increase the temperature of 1 kilogram of water by 1 °C. One Calorie is equivalent to 1,000 calories, or 4.2 kilojoules.
What is a chemical bond?
A chemical bond is an attractive force between atoms. Atoms are the basic building blocks that make up everything in life. This includes the trees, the air, the pencil, the computer, and even yourself! Atoms are made up of a negatively charged electron cloud that surrounds a nucleus which stores positively charged protons and neutral neutrons. Chemical bonds form when atoms share or transfer electrons to try to be as stable as possible.
What is chemical potential energy?
Chemical potential energy is energy related to the position and composition of atoms. It is stored in the chemical bonds of atoms. When energy is absorbed, an endothermic reaction occurs, like when a chemical bond breaks, because it takes energy to break the bond. Alternatively, stored energy can be released as heat, known as an exothermic reaction, like when a chemical bond forms. For example, in an exothermic reaction like burning fuel, molecules form strong bonds, the chemical potential energy in the bonds are converted into heat as well as light.
What is a combustion reaction?
A combustion reaction is an exothermic chemical reaction where compounds react with oxygen (O2) to form carbon dioxide (CO2) and water (H2O). A compound is a combination of two or more elements bonded together like CH4. If a compound reacts with oxygen in a combustion reaction to form carbon dioxide and water, heat is also released. This heat can be released in the form of light, like in the case of a fire where fuel undergoes combustion.
What is a calorimeter?
A calorimeter is a tool that measures the heat of chemical reactions. Heat should flow within the calorimeter but not from the calorimeter to the environment. In order for heat not to escape to the outside surroundings and to contain the heat within the system, calorimeters should be made with an insulating material like a Styrofoam cup with a lid filled with water and a reactant. This type of calorimeter is called a coffee cup calorimeter and frequently used in high schools to demonstrate calorimeters. A thermometer is inserted through the lid and records the temperature of the water and reactant at various times. The change in temperature is used to calculate the heat flow as energy is exchanged. An example of the exchange would be measuring ice melting. Energy is lost to the water thus becoming colder, but energy is gained to the ice, which causes it to melt. For more accurate results, scientists use bomb calorimeters. The bomb calorimeter has a reaction chamber that withstands high pressure and heat. The reaction chamber is surrounded by water where the thermometer is located to record the heat released from the chamber.
How is the number of chemical bonds and chemical potential energy related?
The chemical potential energy is directly related to the number of chemical bonds. Chemical potential energy is stored in chemical bonds. Generally, if there are more chemical bonds, there is more chemical potential energy. If there are fewer chemical bonds, there is less chemical potential energy.
What is a chemical reaction?
Imagine you're baking a cake. You mix flour, sugar, eggs, and butter. Those ingredients are like the "before" team. When you bake them, something amazing happens! They transform into a delicious cake. That transformation is a chemical reaction! A chemical reaction is when substances change into completely new substances. Sometimes it's obvious, like when wood burns and turns to ash. Other times, it's less obvious, like when iron rusts. But always, new stuff is created. Chemical reactions are happening all around us, making our world exciting!
What are indicators of a chemical reaction?
How do you know a chemical reaction has happened? Look for clues! Did the color change? Did bubbles suddenly appear? Did it get hot or cold? These are indicators! A new smell can also mean a chemical reaction. Basically, if something new appears that wasn't there at the start, or if something disappears that was there, a chemical reaction probably took place. These signs are like detective clues helping us understand the world around us!
What are reactants and products?
In a chemical reaction, we have "before" and "after" teams. The "before" team is called the reactants. They're the ingredients you start with, like flour and sugar when baking a cake. The "after" team is called the products. They're the new substances formed, like the cake itself! Reactants interact and rearrange their atoms during the reaction. Think of it like building with LEGOs – the individual bricks are the reactants, and the finished LEGO creation is the product. Chemical equations use symbols to show reactants on the left and products on the right, separated by an arrow that shows the direction of the change.
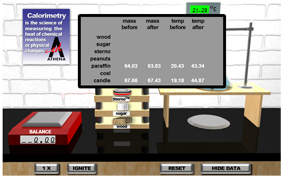 Which has more energy per unit mass stored in its chemical bonds: a candle, a peanut, or a candy bar? In this simulation you’ll investigate how the amount of energy stored in different substances can be determined using a tool called a calorimeter. You’ll expose each substance to a controlled combustion reaction to measure the energy released when the substance is burned. Are you fired up? Well, let’s get started.
Which has more energy per unit mass stored in its chemical bonds: a candle, a peanut, or a candy bar? In this simulation you’ll investigate how the amount of energy stored in different substances can be determined using a tool called a calorimeter. You’ll expose each substance to a controlled combustion reaction to measure the energy released when the substance is burned. Are you fired up? Well, let’s get started.

