What is hemoglobin?
Hemoglobin is a large complex molecule consisting of two pairs of protein globin chains: two called “alpha” (α) and two that are “non-alpha” (beta β in adult hemoglobin, gamma γ in fetal hemoglobin, for example), all of which associate but are not actually covalently attached to each other. Each of the four globin chains holds a protoporphyrin ring called heme with one iron ion (Fe+2) at its center. Together, it is their structure which affects how hemoglobin does its functions: (1) holding and releasing oxygen and (2) buffering the pH of the fluid inside the red blood cell.
How are oxyhemoglobin and deoxyhemoglobin structurally different?
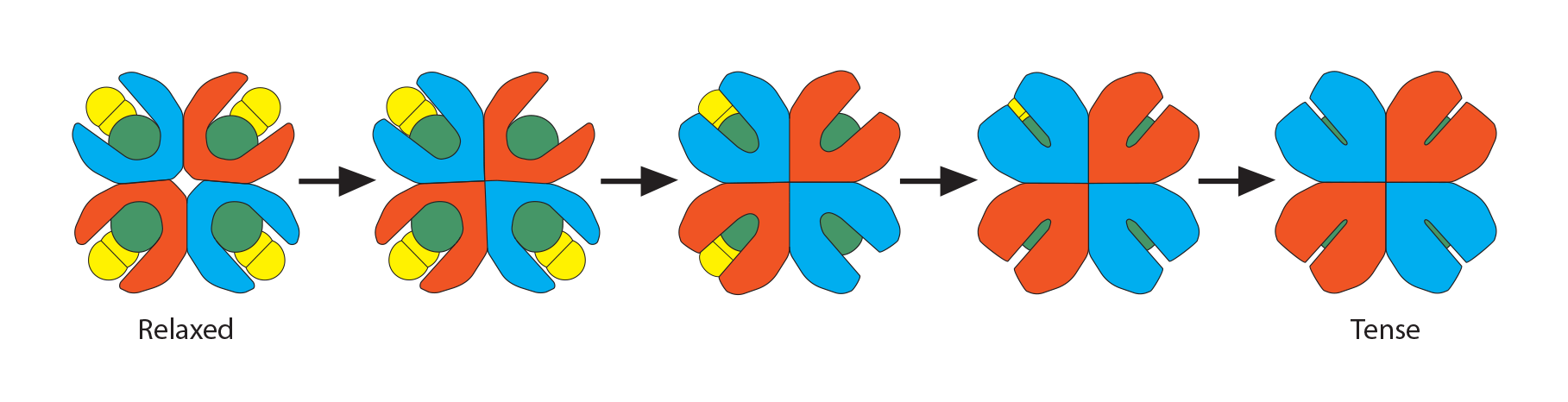
As the names imply, “oxyhemoglobin” is the form of hemoglobin which is binding oxygen, while “deoxyhemoglobin” has released the oxygen. There are multiple chemical and physical realities which allow hemoglobin to move rapidly from one form to the other and at the most physiologically appropriate time. In this illustration of an adult hemoglobin, the red structures represent the alpha chains, the blue structures are the beta chains, and the green structure found in each globin chain’s groove is its heme group (protoporphyrin plus central Fe2+). In oxyhemoglobin, the oxygen molecule is yellow. At this point, notice that the deoxyhemoglobin has a tighter, more compact shape than oxyhemoglobin. For this reason, deoxyhemoglobin is called the “T” (“tense”) formation while oxyhemoglobin is called the “R” (relaxed”) formation. Note also the opening in the hemoglobin configuration in the oxyhemoglobin form.
What are some factors that affect the tense or relaxed state of hemoglobin?
These two states are related to the 3-dimensional orientation of each globin chain to its neighbors. They reflect three critical sequential processes that, together, define the “cooperativity” property of hemoglobin. Prepare to be amazed since all of this happens rapidly, every second of your life! We will start at the tissues.
Process #1: At the tissues, the red blood cells travelling through the capillaries carry oxyhemoglobin, which has an oxygen molecule associated with the iron in each of the four heme groups (one iron is illustrated here). As CO2 leaves the tissue cells and enters first into the plasma, about 10% of the gas dissolves there.
The remaining 90% will enter the red blood cell and find hemoglobin. 20% of that CO2 will react with the amino terminus of each globin chain, forming a carbamino NHCOO- group. This formation of an ionized group interrupts the stable globin chain structure, contributing to a 3-dimensional movement of that globin chain in relationship to its neighbor.
Process #2: 70% of the CO2 entering the red blood cell will react with the intracellular water, using the catalytic activity of carbonic anhydrase, and will form carbonic acid (H2CO3), which then dissociates into bicarbonate (HCO3-) and hydrogen (H+) ions.
The bicarbonate ion will enter the plasma and the hydrogen ion will react with histidines and arginines in the globin chain. The most critical of these reactions happens with the histidine coordinating the heme group’s Fe2+….
…….which at this moment is (1) in a planar orientation to its heme……..
and (2) is pulling on the globin’s histidine…………..
and (3) is non-covalently attached to O2.
Because of this unique arrangement, when the first oxygen molecule leaves its Fe2+, just from the significant O2 concentration gradient between red blood cell and tissue cell, the iron will move out of its planar orientation………..
……..pulling less on the histidine coordinating it……
……shifting the whole globin chain enough that the configuration becomes more tense again, which then makes it harder for the other globin chains in hemoglobin to hold their oxygens. One-by-one, each globin chain’s heme Fe2+ releases its oxygens, resulting in the final tense, deoxygenated hemoglobin form.
Process #3: While all the above is happening, there is also another molecule in the red blood cell’s cytoplasm, ready to play its role: 2,3-bisphosphoglycerate (2,3-BPG). This small molecule (about 9 angstroms in size) is an intermediate in the red blood cell’s glucose metabolism, and there is a delicate balance between generating ATP needed for the cell to survive and using this alternate pathway to make this small molecule that is intimately involved in hemoglobin’s oxygen-carrying function. As the initial globin conformation changes happen, described in processes 1 and 2 above, the globin chains move closer to each other, forming salt bridges, thus losing their overall “relaxed” formation. This happens one globin chain at a time until all the oxygen molecules have been released.
Recall a unique conformational change that happens as “relaxed” oxyhemoglobin releases its oxygens and becomes “tense” deoxyhemoglobin. The opening at the core of the globin configuration is getting smaller, and just wide enough for 2,3-BPG (red bar) to fit into. This stabilizes the tense form as well as makes it even easier for the last oxygens to be released.
)
These three processes go in reverse order when the red blood cell carrying deoxygenated hemoglobin returns to the alveolus.
Higher oxygen concentration in that alveolus will make oxygen molecules move from the alveolus into the plasma and then into the red blood cell to encounter hemoglobin. At first, the tense form of hemoglobin resists having oxygen enter that first crevice containing the waiting porphyrin ring and Fe2, which is in a domed plane and attached to a histidine.
When that first O2 interacts with the first iron, the iron will move into the plane of the heme and pull on the histidine, which then makes the whole globin chain change its conformation.
This movement of the hemoglobin conformation makes it easier for the second, then the third, then the fourth oxygen molecule to bind to the other irons in hemoglobin. As the hemoglobin assumes a more relaxed conformation, the 2,3-BPG can no longer fit into the widening opening within the hemoglobin, and its departure facilitates an even easier binding of oxygen to the remaining heme groups and a final relaxed oxyhemoglobin conformation.
Simultaneously, the buffered hydrogen ion associated with that histidine is released, bicarbonate ions enter the cell and combine with it to form H2CO3, which dissociates into CO2 and water. The CO2 joins with the dissolved CO2 in the cytoplasm, leaves the red blood cell, joins the CO2 dissolved in the plasma, and finally enters the alveolus for expiration.
All this movement and buffering is happening within the 250 milliseconds it takes for a red blood cell to pass through the lung capillaries—and again at the tissue capillaries—pretty amazing!
What is 2,3-BPG?
2,3-bisphosphoglycerate (2,3-BPG) is sometimes labeled 2,3-diphosphoglycerate (2,3-DPG). This small molecule (about 9 angstroms in size) is an intermediate in the red blood cell’s glucose metabolism via the Luebering-Rapoport pathway. The red blood cell sacrifices the production of one ATP to produce this 2,3-BPG via a bisphosphoglycerate mutase which moves a phosphate group from carbon 1 to carbon 2. This creates a molecule which fits between the beta chains of hemoglobin when hemoglobin is deoxygenated, thus stabilizing a “tense” formation. When hemoglobin becomes oxygenated at the lungs, the addition of oxygen molecules, one at a time, causes a “relaxation” of the hemoglobin conformation, causing the 2,3-DPG to be ejected.
The placement of 2,3-BPG causes oxygen to be released from hemoglobin more easily at the tissues, and the removal of 2,3-BPG at the lungs makes the addition of oxygen molecules 3 and 4 happen more easily than with the first two oxygen molecules. Thus, its binding preferentially to deoxygenated hemoglobin reduces the affinity of oxygen. Therefore, high levels of 2,3-BPG are associated with the tense form of hemoglobin (deoxygenated), while low levels will be associated with the relaxed form (oxygenated). This phenomenon explains why banked blood has a time-limited usefulness. As the concentration of 2,3-BPG decreases, due to the decrease in the regular glycolytic pathway as glucose levels in the blood are used up, the oxygen will remain bound to the hemoglobin and be unavailable when transfused.
What does a person’s oxygen dissociation curve indicate?
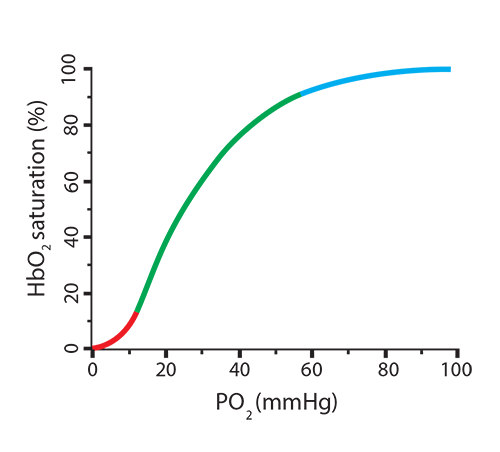
It is easy to visualize the relationship between oxygen concentration and the percentage of oxygen saturation of hemoglobin by using an oxygen dissociation curve. As the partial pressure of oxygen (PO2) increases, the amount of oxygen associated with hemoglobin (oxyhemoglobin) also increases. Note something important, though, in the shape of the curve—it is sigmoidal (shaped like an “S”) rather than linear. At the lowest O2 concentrations, very little binding is happening (red). In the tense conformation of hemoglobin (with 2,3-BPG in place), the iron is out of the plane of the heme group and is attached to the histidine group. This conformation resists having oxygen enter the binding crevice and associate with iron. Once this first oxygen binds, however, iron moves into the heme plane, relaxing its hold on the histidine, and the globin chain will shift position. This allows the second, third, and fourth oxygen molecules to bind more readily (green), one-at-a-time, changing the hemoglobin conformation enough to eject the 2,3-BPG, until the hemoglobin is 100% saturated (blue).
)
So what can make hemoglobin release more oxygen in the tissues when needed, such as during exercise? A review of those three processes above (form a carbamino, buffer the histidine holding iron, insert 2,3-BPG) can provide some of the answer, since we need situations that will cause hemoglobin to morph from its saturated oxyhemoglobin form (“relaxed”) to its deoxyhemoglobin form (“tense”).
- Higher CO2 coming into the red blood cell will generate more NHCOO- groups, making the globin chain move, lowering O2 affinity ☑
- Higher CO2 coming into the red blood cell will generate more H+ to be buffered by histidine, making the globin chain move, lowering O2 affinity ☑
- Increased 2,3-BPG binding will decrease O2 affinity for hemoglobin ☑
In the new scenario, the hemoglobin went from releasing 20% of its oxygen (going from 100% saturation to 80%) to now releasing 50% (going from 100% saturation to 50%). This is called a shift to the right, indicating a more favorable environment for hemoglobin to release its oxygen. Here is a way to remember this… Right Releases. Conditions where this will happen are:
What can make hemoglobin hold onto the oxygen, even at low PO2 levels? Once more, a review of those three processes above (remove carbamino, remove extra H+ from histidine holding iron, remove 2,3-BPG) can provide some of the answer, since we need situations that will cause hemoglobin to remain at a higher saturation level.
- Lower CO2 coming into the red blood cell will generate fewer NHCOO- groups, making the globin chain move less, maintaining O2 affinity ☑
- Lower CO2 coming into the red blood cell will generate fewer H+ to be buffered by histidine, making the globin chain move less, maintaining O2 affinity ☑
- Decreased 2,3-BPG binding will increase O2 affinity for hemoglobin ☑
In the new scenario, the hemoglobin went from releasing 20% of its oxygen (going from 100% saturation to 80%) to now releasing only 8% (going from 100% saturation to 92%). This is called a shift to the left, indicating a less favorable environment for hemoglobin to release its oxygen. Here is a way to remember this… Left Latches on. Conditions where this will happen are:
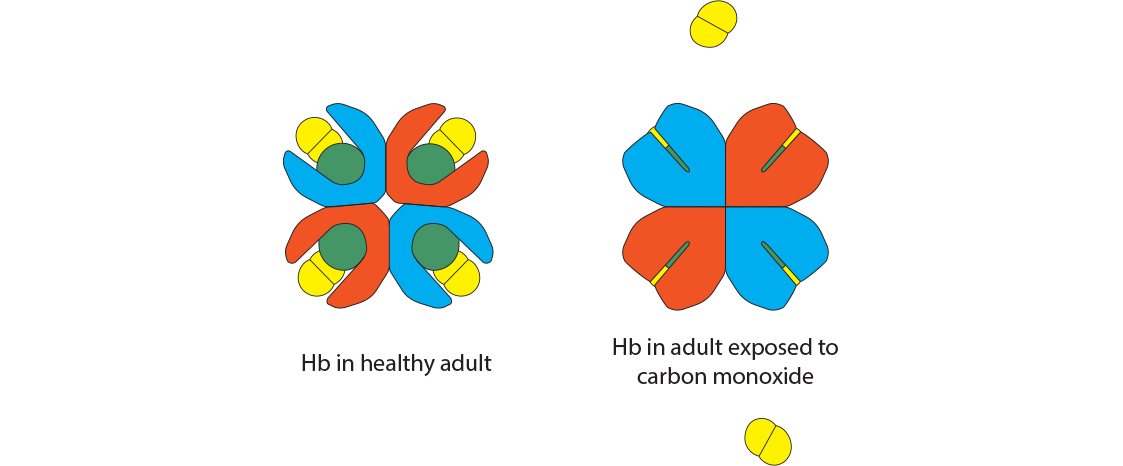
How does carbon monoxide affect hemoglobin?
Carbon monoxide is a colorless, odorless gas released most often through incomplete combustion, as in fires. It has an affinity for hemoglobin that is 300 times that of oxygen. Binding of CO places the hemoglobin conformation into the tense state, preventing complete O2 saturation from happening. The resulting hemoglobin form is called carboxyhemoglobin. Be careful not to think of this as hemoglobin bound to carbon dioxide, which would be called “carbaminohemoglobin.” Also note that the iron valence in carbon monoxide is still Fe2+, so if caught in time, oxygen can displace the CO.
The oxygen dissociation curve changes dramatically with even the smallest exposure to carbon monoxide. Treatment with 100% oxygen early in the exposure will still require about 74 minutes to displace only half of the bound CO. Notice that CO produces a shift to the left since its presence makes it difficult for any bound oxygen to be released, while preventing additional oxygen from binding.
What are some factors that impact the amount of oxygen in a person’s bloodstream?
Oxygen reaching the bloodstream relies on:
- A sufficient source of oxygen. Air at all levels of altitude has 21% O2, but high altitudes would have less partial pressure to drive the O2 into the red blood cells from the alveoli.
- Functioning alveoli. Diseases where fewer alveoli are available for gas exchange will affect oxygen saturation.
- Normal thickness of the pathway through alveolar and capillary lining cells, connective tissue, basement membranes. Any change to this thickness, such as increased sticky mucus from asthma, can make it more difficult for O2 to cross from the alveolus to the red blood cell.
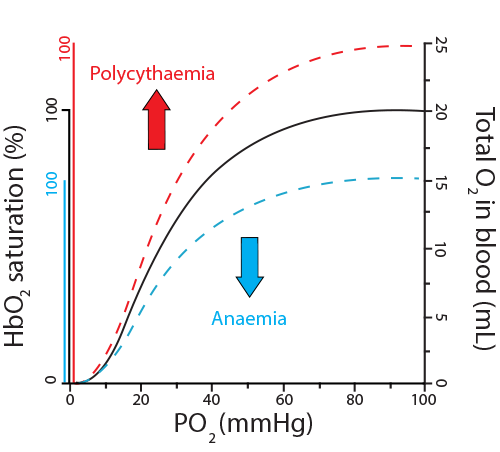
- Sufficient red blood cells and hemoglobin. Anemias affecting either the red blood cell count or the hemoglobin concentration can result in less oxygen in the bloodstream. Conversely, individuals with conditions producing too many red blood cells (polycythemia) will have higher total oxygen content in their bloodstreams. Notice, however, that both anemias and polycythemia still show a sigmoidal dissociation curve, with “saturation” still occurring.
)
- Normal heart function. Since even normal levels of oxygen and hemoglobin are limited in circulating oxygen if they are not kept moving in the circulation, any cardiac dysfunction may lead to lowered levels of oxygen in the blood stream.
 Inside red blood cells you will find hemoglobin, which has a critical role to play in carrying and releasing oxygen. Your challenge in this module includes describing the structure and function of hemoglobin in all of its biologically active forms, manipulating the amino acid histidine in hemoglobin to make hemoglobin become an acid or base, correlating hemoglobin’s “tense” or “relaxed” state and its ability to hold or release oxygen, predicting the effect of 2,3-DPG and carbon monoxide on hemoglobin’s structure and function, and describing the role that oxygen plays in all of this.
Inside red blood cells you will find hemoglobin, which has a critical role to play in carrying and releasing oxygen. Your challenge in this module includes describing the structure and function of hemoglobin in all of its biologically active forms, manipulating the amino acid histidine in hemoglobin to make hemoglobin become an acid or base, correlating hemoglobin’s “tense” or “relaxed” state and its ability to hold or release oxygen, predicting the effect of 2,3-DPG and carbon monoxide on hemoglobin’s structure and function, and describing the role that oxygen plays in all of this.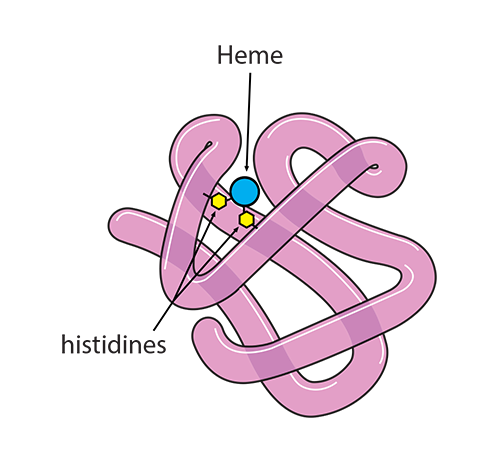)
)
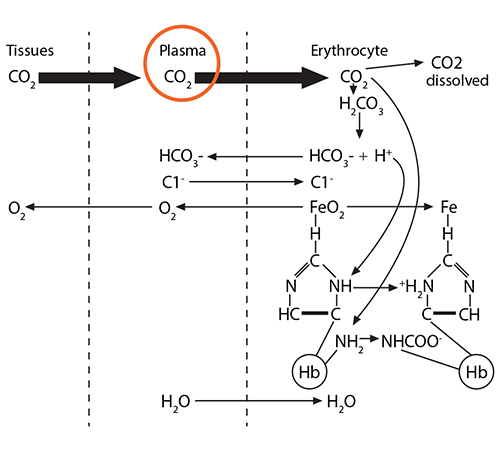) The remaining 90% will enter the red blood cell and find hemoglobin. 20% of that CO2 will react with the amino terminus of each globin chain, forming a carbamino NHCOO- group. This formation of an ionized group interrupts the stable globin chain structure, contributing to a 3-dimensional movement of that globin chain in relationship to its neighbor.
The remaining 90% will enter the red blood cell and find hemoglobin. 20% of that CO2 will react with the amino terminus of each globin chain, forming a carbamino NHCOO- group. This formation of an ionized group interrupts the stable globin chain structure, contributing to a 3-dimensional movement of that globin chain in relationship to its neighbor.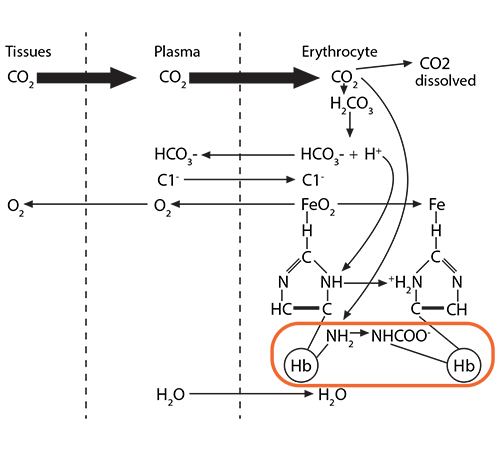) Process #2: 70% of the CO2 entering the red blood cell will react with the intracellular water, using the catalytic activity of carbonic anhydrase, and will form carbonic acid (H2CO3), which then dissociates into bicarbonate (HCO3-) and hydrogen (H+) ions.
Process #2: 70% of the CO2 entering the red blood cell will react with the intracellular water, using the catalytic activity of carbonic anhydrase, and will form carbonic acid (H2CO3), which then dissociates into bicarbonate (HCO3-) and hydrogen (H+) ions.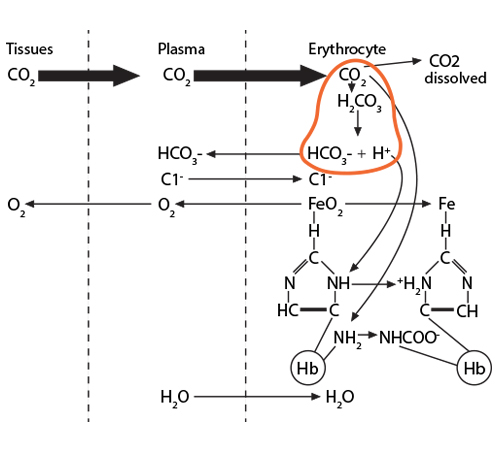) The bicarbonate ion will enter the plasma and the hydrogen ion will react with histidines and arginines in the globin chain. The most critical of these reactions happens with the histidine coordinating the heme group’s Fe2+….
The bicarbonate ion will enter the plasma and the hydrogen ion will react with histidines and arginines in the globin chain. The most critical of these reactions happens with the histidine coordinating the heme group’s Fe2+….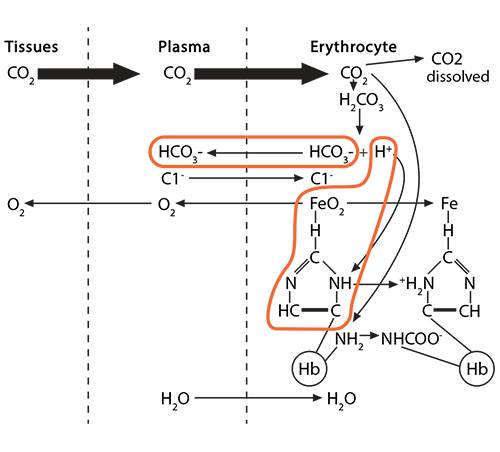) …….which at this moment is (1) in a planar orientation to its heme……..
…….which at this moment is (1) in a planar orientation to its heme……..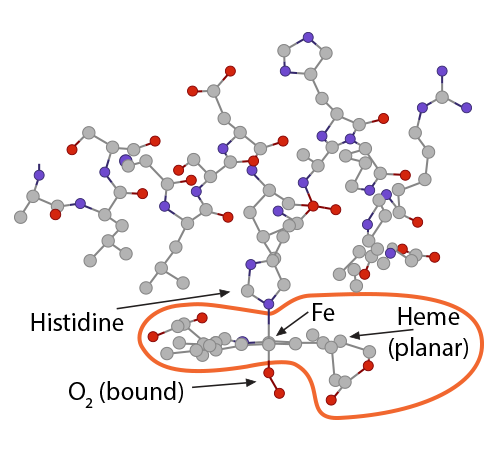) and (2) is pulling on the globin’s histidine…………..
and (2) is pulling on the globin’s histidine…………..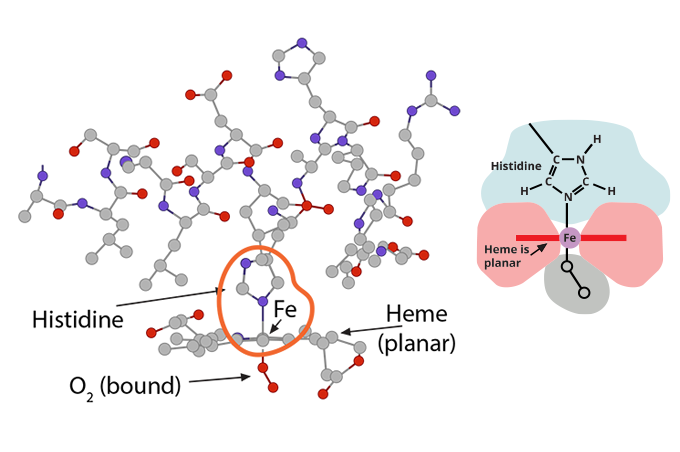) and (3) is non-covalently attached to O2.
and (3) is non-covalently attached to O2.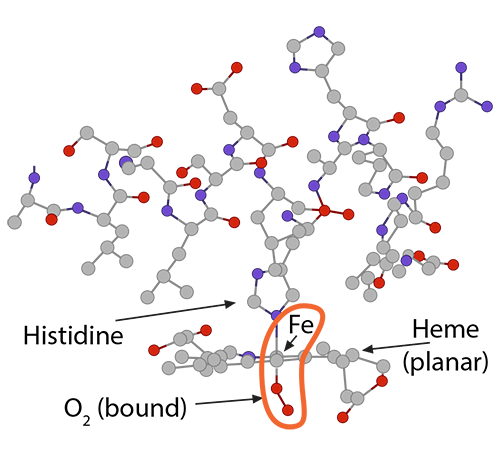) Because of this unique arrangement, when the first oxygen molecule leaves its Fe2+, just from the significant O2 concentration gradient between red blood cell and tissue cell, the iron will move out of its planar orientation………..
Because of this unique arrangement, when the first oxygen molecule leaves its Fe2+, just from the significant O2 concentration gradient between red blood cell and tissue cell, the iron will move out of its planar orientation………..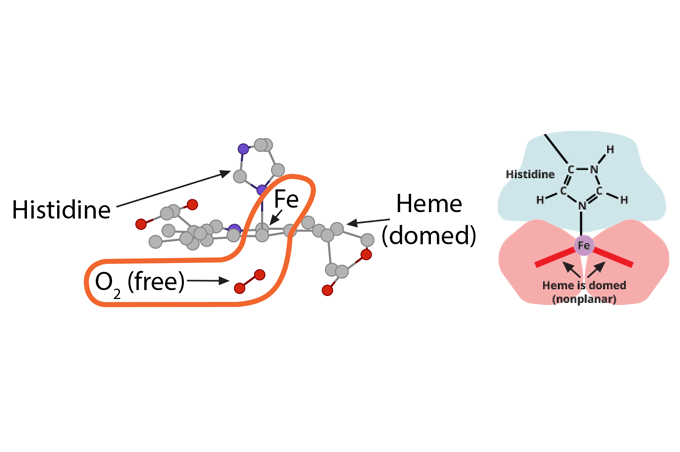) ……..pulling less on the histidine coordinating it……
……..pulling less on the histidine coordinating it……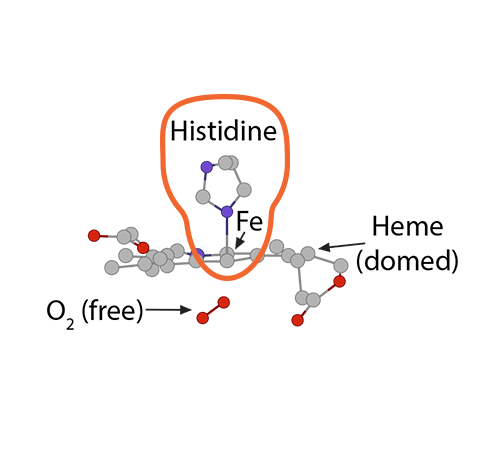) ……shifting the whole globin chain enough that the configuration becomes more tense again, which then makes it harder for the other globin chains in hemoglobin to hold their oxygens. One-by-one, each globin chain’s heme Fe2+ releases its oxygens, resulting in the final tense, deoxygenated hemoglobin form.
……shifting the whole globin chain enough that the configuration becomes more tense again, which then makes it harder for the other globin chains in hemoglobin to hold their oxygens. One-by-one, each globin chain’s heme Fe2+ releases its oxygens, resulting in the final tense, deoxygenated hemoglobin form.) These three processes go in reverse order when the red blood cell carrying deoxygenated hemoglobin returns to the alveolus.
These three processes go in reverse order when the red blood cell carrying deoxygenated hemoglobin returns to the alveolus.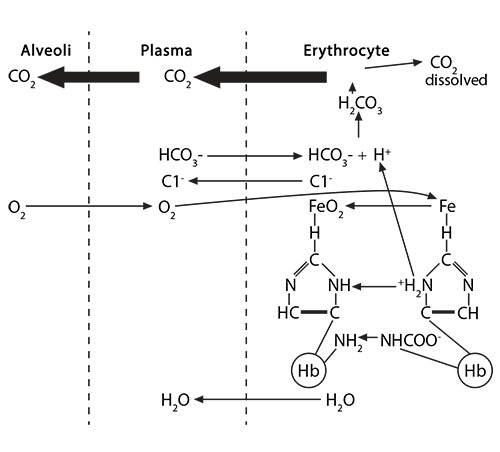) Higher oxygen concentration in that alveolus will make oxygen molecules move from the alveolus into the plasma and then into the red blood cell to encounter hemoglobin. At first, the tense form of hemoglobin resists having oxygen enter that first crevice containing the waiting porphyrin ring and Fe2, which is in a domed plane and attached to a histidine.
Higher oxygen concentration in that alveolus will make oxygen molecules move from the alveolus into the plasma and then into the red blood cell to encounter hemoglobin. At first, the tense form of hemoglobin resists having oxygen enter that first crevice containing the waiting porphyrin ring and Fe2, which is in a domed plane and attached to a histidine.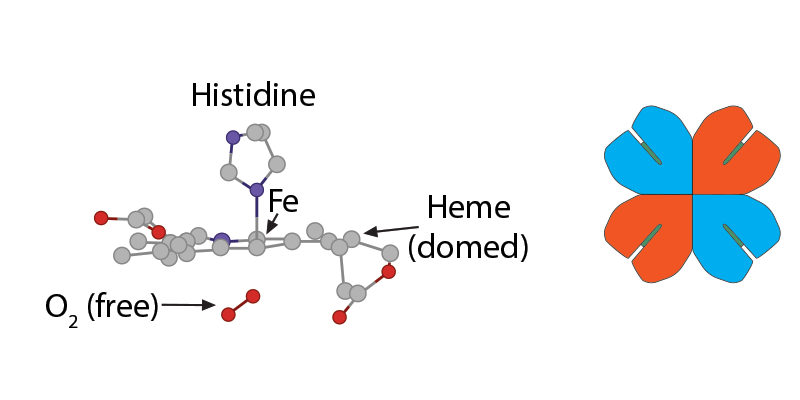) When that first O2 interacts with the first iron, the iron will move into the plane of the heme and pull on the histidine, which then makes the whole globin chain change its conformation.
When that first O2 interacts with the first iron, the iron will move into the plane of the heme and pull on the histidine, which then makes the whole globin chain change its conformation.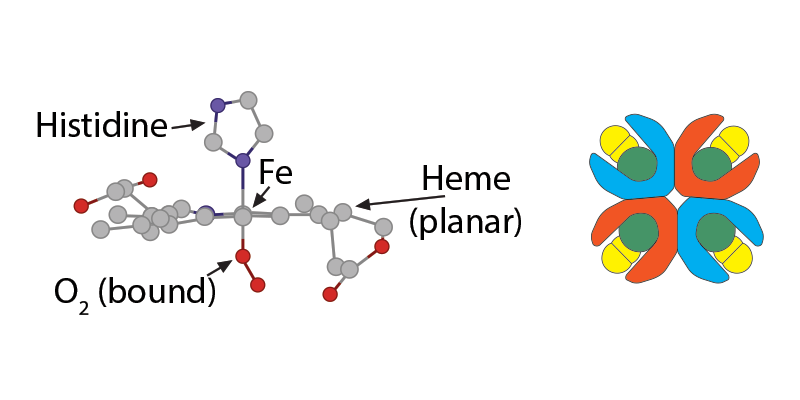) This movement of the hemoglobin conformation makes it easier for the second, then the third, then the fourth oxygen molecule to bind to the other irons in hemoglobin. As the hemoglobin assumes a more relaxed conformation, the 2,3-BPG can no longer fit into the widening opening within the hemoglobin, and its departure facilitates an even easier binding of oxygen to the remaining heme groups and a final relaxed oxyhemoglobin conformation.
This movement of the hemoglobin conformation makes it easier for the second, then the third, then the fourth oxygen molecule to bind to the other irons in hemoglobin. As the hemoglobin assumes a more relaxed conformation, the 2,3-BPG can no longer fit into the widening opening within the hemoglobin, and its departure facilitates an even easier binding of oxygen to the remaining heme groups and a final relaxed oxyhemoglobin conformation.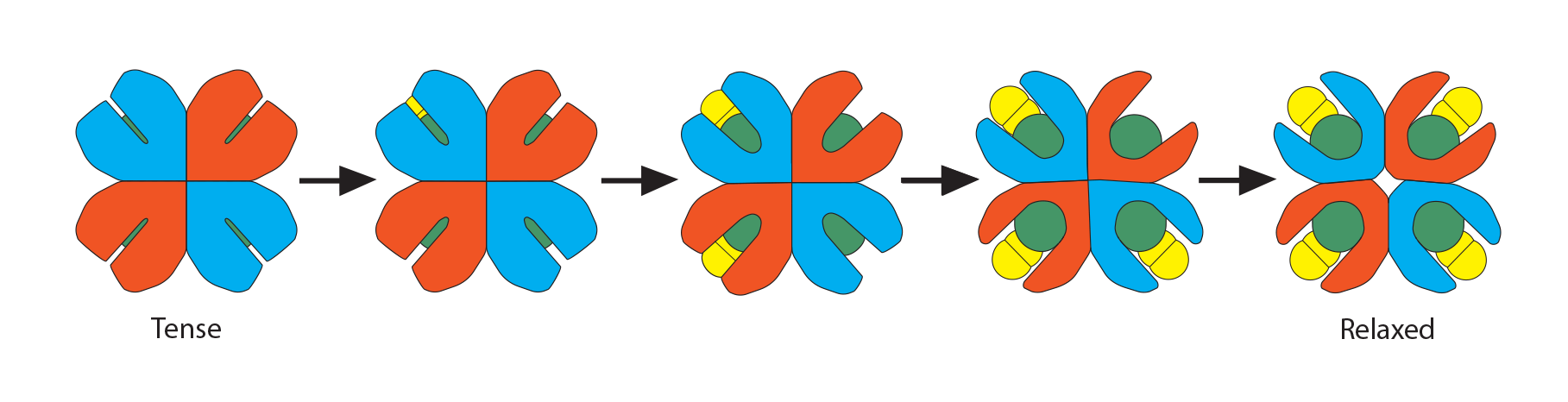) Simultaneously, the buffered hydrogen ion associated with that histidine is released, bicarbonate ions enter the cell and combine with it to form H2CO3, which dissociates into CO2 and water. The CO2 joins with the dissolved CO2 in the cytoplasm, leaves the red blood cell, joins the CO2 dissolved in the plasma, and finally enters the alveolus for expiration.
Simultaneously, the buffered hydrogen ion associated with that histidine is released, bicarbonate ions enter the cell and combine with it to form H2CO3, which dissociates into CO2 and water. The CO2 joins with the dissolved CO2 in the cytoplasm, leaves the red blood cell, joins the CO2 dissolved in the plasma, and finally enters the alveolus for expiration.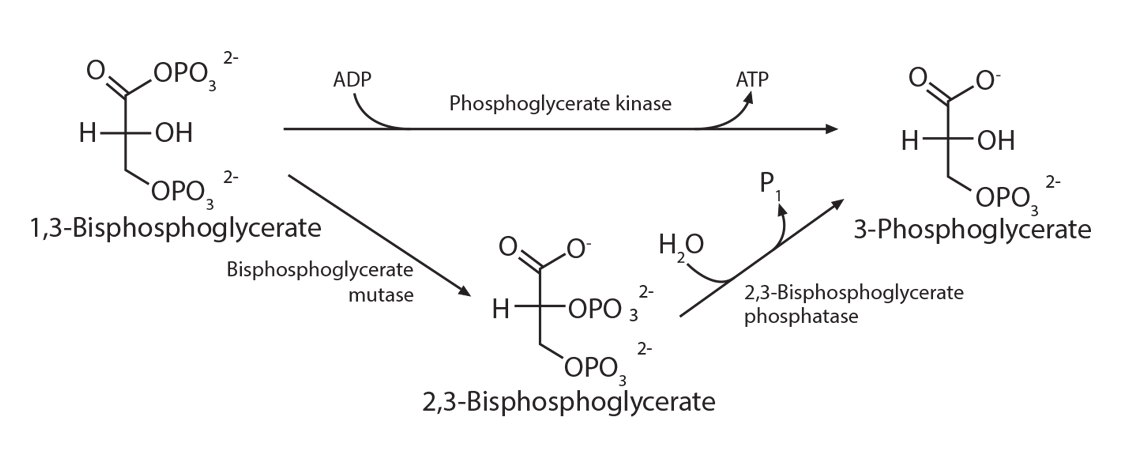) The placement of 2,3-BPG causes oxygen to be released from hemoglobin more easily at the tissues, and the removal of 2,3-BPG at the lungs makes the addition of oxygen molecules 3 and 4 happen more easily than with the first two oxygen molecules. Thus, its binding preferentially to deoxygenated hemoglobin reduces the affinity of oxygen. Therefore, high levels of 2,3-BPG are associated with the tense form of hemoglobin (deoxygenated), while low levels will be associated with the relaxed form (oxygenated). This phenomenon explains why banked blood has a time-limited usefulness. As the concentration of 2,3-BPG decreases, due to the decrease in the regular glycolytic pathway as glucose levels in the blood are used up, the oxygen will remain bound to the hemoglobin and be unavailable when transfused.
The placement of 2,3-BPG causes oxygen to be released from hemoglobin more easily at the tissues, and the removal of 2,3-BPG at the lungs makes the addition of oxygen molecules 3 and 4 happen more easily than with the first two oxygen molecules. Thus, its binding preferentially to deoxygenated hemoglobin reduces the affinity of oxygen. Therefore, high levels of 2,3-BPG are associated with the tense form of hemoglobin (deoxygenated), while low levels will be associated with the relaxed form (oxygenated). This phenomenon explains why banked blood has a time-limited usefulness. As the concentration of 2,3-BPG decreases, due to the decrease in the regular glycolytic pathway as glucose levels in the blood are used up, the oxygen will remain bound to the hemoglobin and be unavailable when transfused.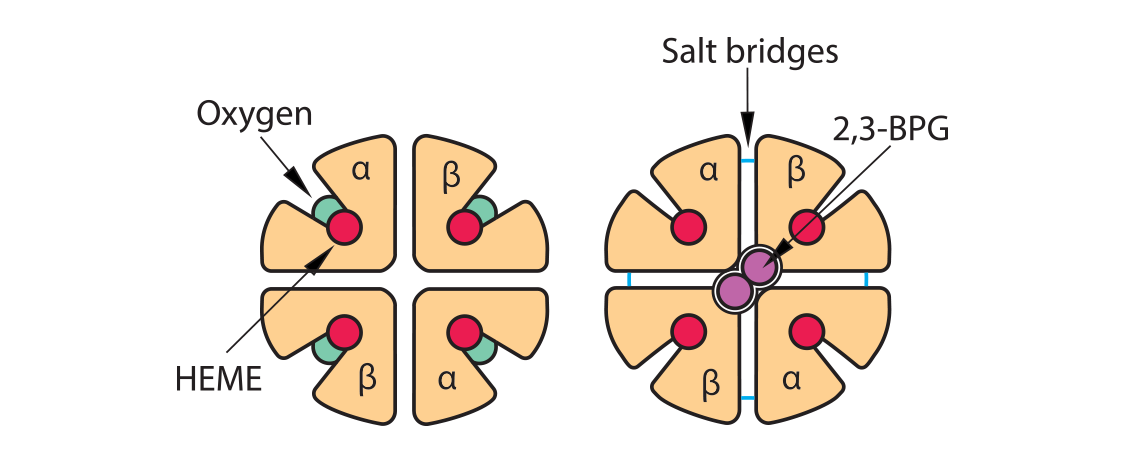)
) So what can make hemoglobin release more oxygen in the tissues when needed, such as during exercise? A review of those three processes above (form a carbamino, buffer the histidine holding iron, insert 2,3-BPG) can provide some of the answer, since we need situations that will cause hemoglobin to morph from its saturated oxyhemoglobin form (“relaxed”) to its deoxyhemoglobin form (“tense”).
So what can make hemoglobin release more oxygen in the tissues when needed, such as during exercise? A review of those three processes above (form a carbamino, buffer the histidine holding iron, insert 2,3-BPG) can provide some of the answer, since we need situations that will cause hemoglobin to morph from its saturated oxyhemoglobin form (“relaxed”) to its deoxyhemoglobin form (“tense”).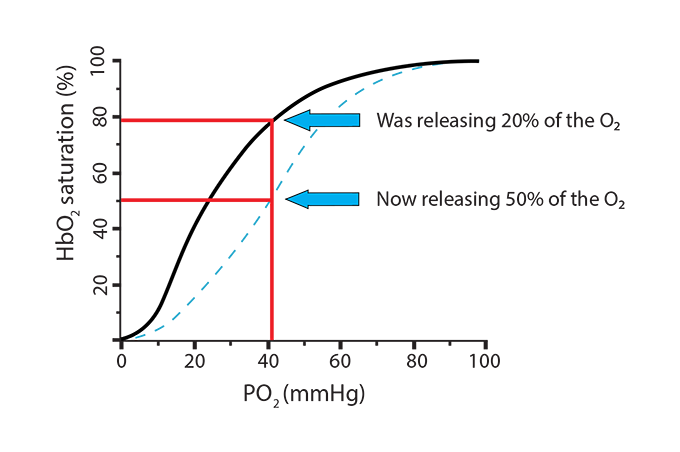) In the new scenario, the hemoglobin went from releasing 20% of its oxygen (going from 100% saturation to 80%) to now releasing 50% (going from 100% saturation to 50%). This is called a shift to the right, indicating a more favorable environment for hemoglobin to release its oxygen. Here is a way to remember this… Right Releases. Conditions where this will happen are:
In the new scenario, the hemoglobin went from releasing 20% of its oxygen (going from 100% saturation to 80%) to now releasing 50% (going from 100% saturation to 50%). This is called a shift to the right, indicating a more favorable environment for hemoglobin to release its oxygen. Here is a way to remember this… Right Releases. Conditions where this will happen are: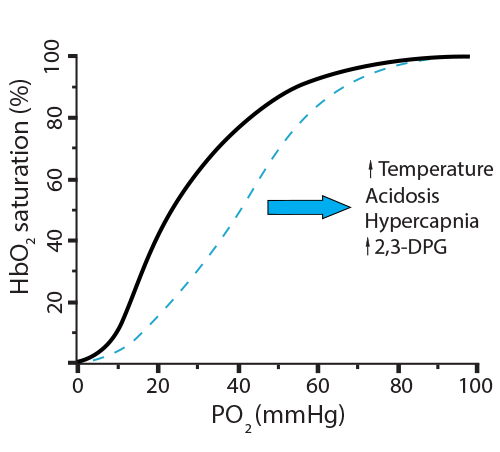) What can make hemoglobin hold onto the oxygen, even at low PO2 levels? Once more, a review of those three processes above (remove carbamino, remove extra H+ from histidine holding iron, remove 2,3-BPG) can provide some of the answer, since we need situations that will cause hemoglobin to remain at a higher saturation level.
What can make hemoglobin hold onto the oxygen, even at low PO2 levels? Once more, a review of those three processes above (remove carbamino, remove extra H+ from histidine holding iron, remove 2,3-BPG) can provide some of the answer, since we need situations that will cause hemoglobin to remain at a higher saturation level.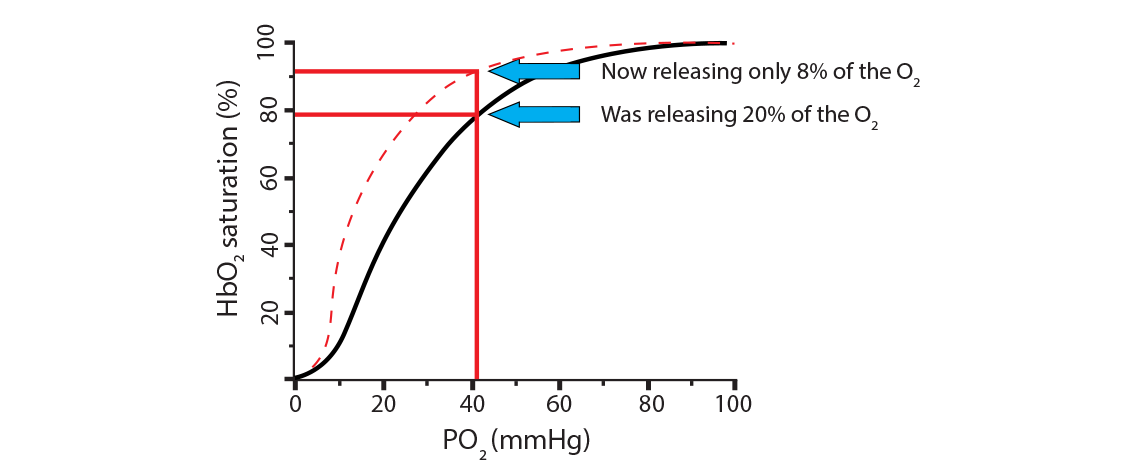) In the new scenario, the hemoglobin went from releasing 20% of its oxygen (going from 100% saturation to 80%) to now releasing only 8% (going from 100% saturation to 92%). This is called a shift to the left, indicating a less favorable environment for hemoglobin to release its oxygen. Here is a way to remember this… Left Latches on. Conditions where this will happen are:
In the new scenario, the hemoglobin went from releasing 20% of its oxygen (going from 100% saturation to 80%) to now releasing only 8% (going from 100% saturation to 92%). This is called a shift to the left, indicating a less favorable environment for hemoglobin to release its oxygen. Here is a way to remember this… Left Latches on. Conditions where this will happen are: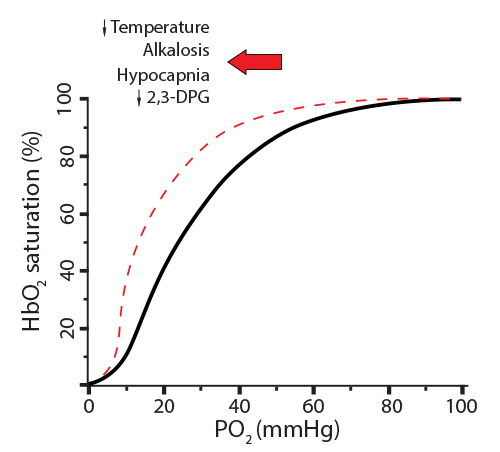)
) The oxygen dissociation curve changes dramatically with even the smallest exposure to carbon monoxide. Treatment with 100% oxygen early in the exposure will still require about 74 minutes to displace only half of the bound CO. Notice that CO produces a shift to the left since its presence makes it difficult for any bound oxygen to be released, while preventing additional oxygen from binding.
The oxygen dissociation curve changes dramatically with even the smallest exposure to carbon monoxide. Treatment with 100% oxygen early in the exposure will still require about 74 minutes to displace only half of the bound CO. Notice that CO produces a shift to the left since its presence makes it difficult for any bound oxygen to be released, while preventing additional oxygen from binding.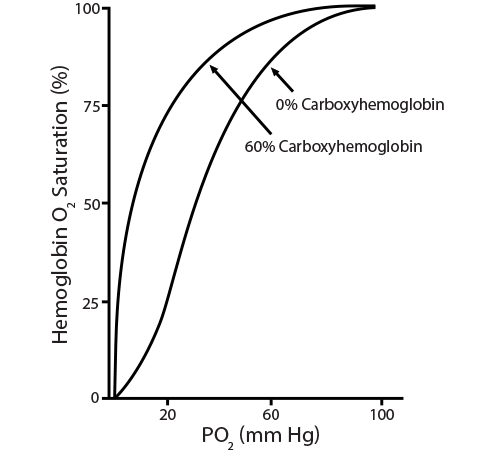)
)


