What are Thiobacillus ferrooxidans?
Thiobacillus ferrooxidans are bacteria that are used in leaching sulfide ores. Leaching is a technique that converts metals into soluble salts. Thiobacillus ferrooxidans can oxidize sulfur compounds as well as oxidize ferrous to ferric iron. This can be useful in biohydrometallurgy, which is a branch of metallurgy that involves the extraction of metals from minerals or solid waste. The bacteria convert the Fe+2 back to Fe+3 so it is recycled into the refinement process; however, the pH of the environment to support the bacteria growth must be maintained around 2. changes in the pH above 2 results in the inhibition of bacterial growth and the decrease in the reduction of iron +3 to +2 since the iron +2 ions will not be recycled back into the process as iron +3 ions.
What is oxidation?
Oxidation is the loss of electrons when substances interact. Examples of oxidation include a cut apple turning brown, a ship rusting, or a copper penny turning green. When the apple gets exposed to the air, the apple cells come into contact with the oxygen. The oxygen causes free radicals on the surface to break away leaving brown spots on the apple. For metals that can rust, the oxygen gets into contact with the metal cells. If there is a layer between the oxygen and surface, this will prevent oxidation. Stainless steel is an example of this. Stainless steel is steel mixed with chromium that has a layer that prevents contact with the oxygen molecules and therefore, keeps the metal from rusting.
What is the difference between ferrous and ferric ions?
Iron is a transition metal that has a positive charge. Because it is a transition metal it can also have multiple oxidation states. Ferrous is in the lower oxidation state with a +2 charge. Ferric is in the higher oxidation state losing one more electron with a +3 charge.
What is an acidic solution?
An acidic solution is a solution where the concentration of hydrogen ions is greater than the concentration of hydroxide ions. Solutions are measured using a pH scale which ranges from 1 to 14. Acidic solutions are less than 7 on a pH scale. Acidic solutions taste sour and react with metals and bases.
What is a buffered solution?
A buffered solution is a solution that resists pH changes when exposed to small amounts of acids or bases. Buffered solutions are created by mixing a weak acid (HA) with large amounts of its conjugate base (A-) in water. If other acids or bases are added to the buffer solution, they will turn the buffer agent into either HA or A- form. Buffer solutions resist changes in pH because of the neutralization process.
What is ore?
Ore is a rock that contains extractable valuable minerals and metals. To get the valuable minerals, ore must first be extracted through mining. Then the ore must be refined to extract the valuable mineral. Metals like iron and copper are in ore.
How is copper metal extracted from ore?
There are three steps in the extraction of copper: mining, extraction, and purification. Copper ore is first mined from the ground. The ore is then changed chemically into the metal through reduction. Because copper has low reactivity, it is easier to extract from ore than aluminum and iron. Depending on the form the copper is in, there are different processes. For copper rich ores, froth flotation concentrates the copper ores then the ores are roasted. The roasted ores are in a more suitable chemical form for reduction. During the reduction process, natural gas is blown onto the molten copper to get rid of oxygen. In the purification process, the impurities are removed through electrolysis. The process creates 99.99% pure copper.
Is copper an important metal?
Copper is a very important metal. Copper is used in many materials in the world today from pipes to electronics. An important characteristic of copper is that it is a good conductor of heat and electricity. All power cords use copper because of its ability to conduct electricity. Because copper is malleable, which means it can be shaped easily, it is also good for electronics. Circuit boards are etched from copper sheets. In addition, pipes are also made from copper. Unlike lead pipes, copper doesn't leach into the water, which makes our drinking water safe.
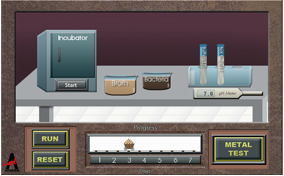 Some predict that the world will run out of copper metal within 25 years. Others say that better mining and extraction techniques will postpone this expected shortfall for many more years and that we are not likely to run out of copper for hundreds of years. No matter who is correct, copper is a valuable element critical for modern life. Your challenge is to determine which conditions result in the greatest production of copper from ore. You’ll use bacteria to aid in the separation of the copper from the ore. Get ready to get some heavy metal...copper.
Some predict that the world will run out of copper metal within 25 years. Others say that better mining and extraction techniques will postpone this expected shortfall for many more years and that we are not likely to run out of copper for hundreds of years. No matter who is correct, copper is a valuable element critical for modern life. Your challenge is to determine which conditions result in the greatest production of copper from ore. You’ll use bacteria to aid in the separation of the copper from the ore. Get ready to get some heavy metal...copper.

